

innovation
Research & Development
- Highly skilled and diverse team comprising of PhD’s, Postgraduates and Chemical Engineers
- Expertise in handling multi-step complex chemistry, extended coupling reactions, stereoselective and chiral complex products
- Full-scale patent evaluation & developing non-infringing processes
- Collaborative Programs under confidentiality agreements
- Development & Validation of test methods and Genotoxic Impurities evaluation
- Air Jet mill Micronizer and Multi-mill
- Purified water system to meet current USP quality
Chemical Research and development (CRD) Infrastructure and capabilities
- Well-equipped R&D centers located at Bhondsi, Gurugram, Haryana, Approved by Department of Scientific and Industrial Research, Ministry of Science & Technology Government of India
- Highly competent Process Chemistry group supported by highly experienced and competent Analytical Development team, Regulatory Affairs, Intellectual Property, engineering Technology development and transfer, Technology improvement and Impurity teams
- The infrastructure includes research and scale-up utilizing latest state of the art micro channel reactor technology, utilization of green technologies like use of enzymes for several products, high pressure hydrogenators, use of software for Design of experiments to focus on Quality by design
- Full-fledged research laboratory for innovation & optimization in API manufacturing process & engineering
Analytical Research and development (ARD) infrastructure and capabilities
- Well equipped with sophisticated advance analytical instrumentations with 21 CFR part 11 compliant
- For development, sample Stability Studies are being performed as per ICH guidelines for following conditions
- Long term Condition 25 C/ 60% RH
- Intermediate Condition 30 C/ 75% RH. Zone IV
- Accelerated Condition 40 C / 75% RH
- Analytical Method Development & Validation as per ICH guideline recommendation to meet worldwide regulatory requirements
Manufacturing operations
- Multi-product cGMP manufacturing units.
- Five powder processing areas as per class ISO 8 (Class 100,000)
- Reaction Temperature ranging from -90°C to +220°C
- Blenders with 300 L to 3000 L volume
- High vacuum distillation at 1 Torr and high-pressure reactions at 20 Bar in combination of 200°C temperature
- Installed reaction capacity of 214,000 L with 68 reactors-31 SSR, 36 GLR, One Cryogenic reactor, ranging from 100 L to 8,000 L
- 15 Centrifuges - (24-60”) stainless steel, halar-coated and peeler
- 18 Dryers : RCVD, VTD, FBD, ANFD, TD and Nauta
- Air Jet mill Micronizer and Multi-mill
- Purified water system to meet current USP quality
The Reactors
The Utility Equipment

Other Equipment
Centrifuges
- Stainless steel
- Halar
- Pressure Nutsche Filters(PNF)
- Peeler & Conventional
Drying Equipment
- Tray
- Vacuum Tray Dryer(VTD)
- Roto cone vacuum dryer(RCVD)
- Fluidized Bed Dryer(FBD)
Milling & Micronization
- Air jet mill micronizer

Blenders
- Octagonal
- Cage
Others
- Dedicated Powder Processing Areas
- Dedicated Purified Water System
- Unit Facilitated with Distillation Columns, Scrubbers, Vacuum Systems, Pressure filters, etc
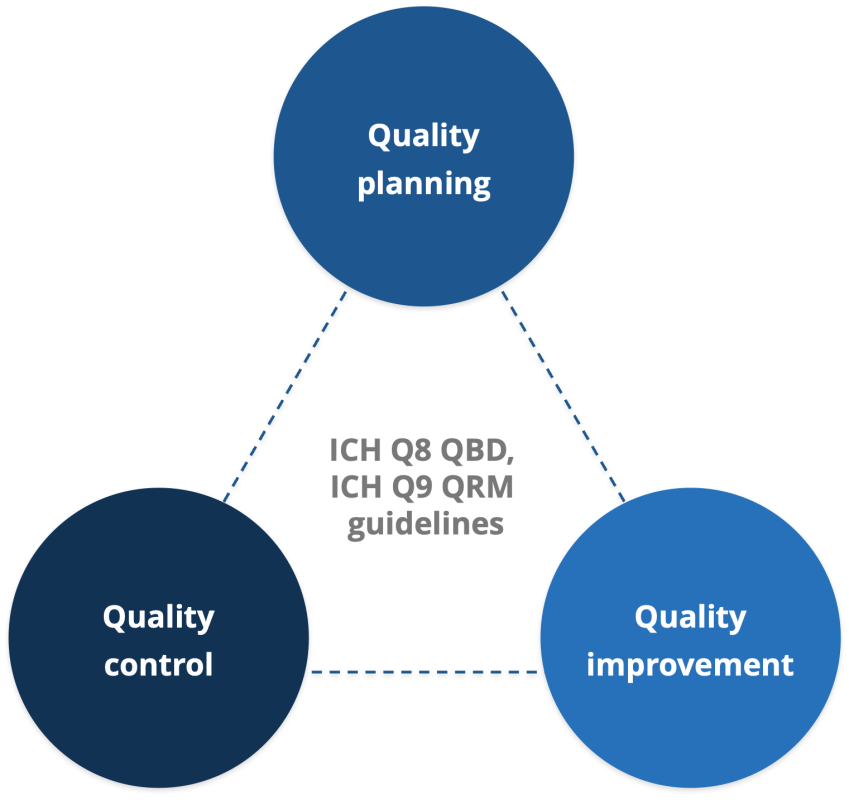
Quality
- We are committed to constantly improve the quality standards of our products and services by implementation of cGMP, up-gradation in technology; imparting training to effectively adapt to newer technologies and high standard
- Well equipped 21 CFR compliant Instrumentation & Microbiology labs
Quality Control
Raw material section
Vendor sample analysis and innovation
Documentation section
Ensuring availability of effective documents on shop floor and retrieval of obsolete documents to prevent usage
Finished goods section
Manufactured products tested for usage and dispatch
Stability section
Stability studies to establish the shelf life and storage condition of active pharmaceutical ingredients and holding time for intermediates
Instrumentation section
Analysis by applicable quality attributes, analytical data provided to RM or FG section for compilation and conclusion
GLP section
Periodic calibration and preventive maintenance of instruments as per schedule
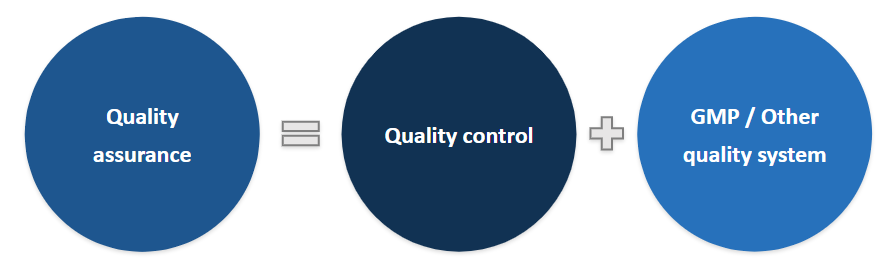
Quality assurance
- It is the totality of the arrangements made with the object ensuring that pharmaceutical products are of the required quality for their intended use
- A dynamic process - journey towards the destination
- The heart and soul of quality control
RESPONSIBILITIES OF QUALITY ASSURANCE
- To achieve the quality objective reliably there must be comprehensively designed and correctly implemented system of Quality Assurance incorporating GMP & quality control
- It should be fully documented & effectiveness monitored
- All parts of quality assurance system should be adequately staffed with competent personnel and should have suitable and sufficient premises, equipment and facilities
Quality Management
- Initiation & Approval of Procedures
- Coordination and approvals of validations and qualifications protocols and reports
- Review and compliance of all Documents
- Change control & Deviation
- On job training and Training on GMP topics
- Investigation (Root Cause analysis) & CAPA
- Handling of Customer complaints
- QA release and Dispatched
- Self inspection
- Vendor qualifications
- Annual product review
- Management reviews
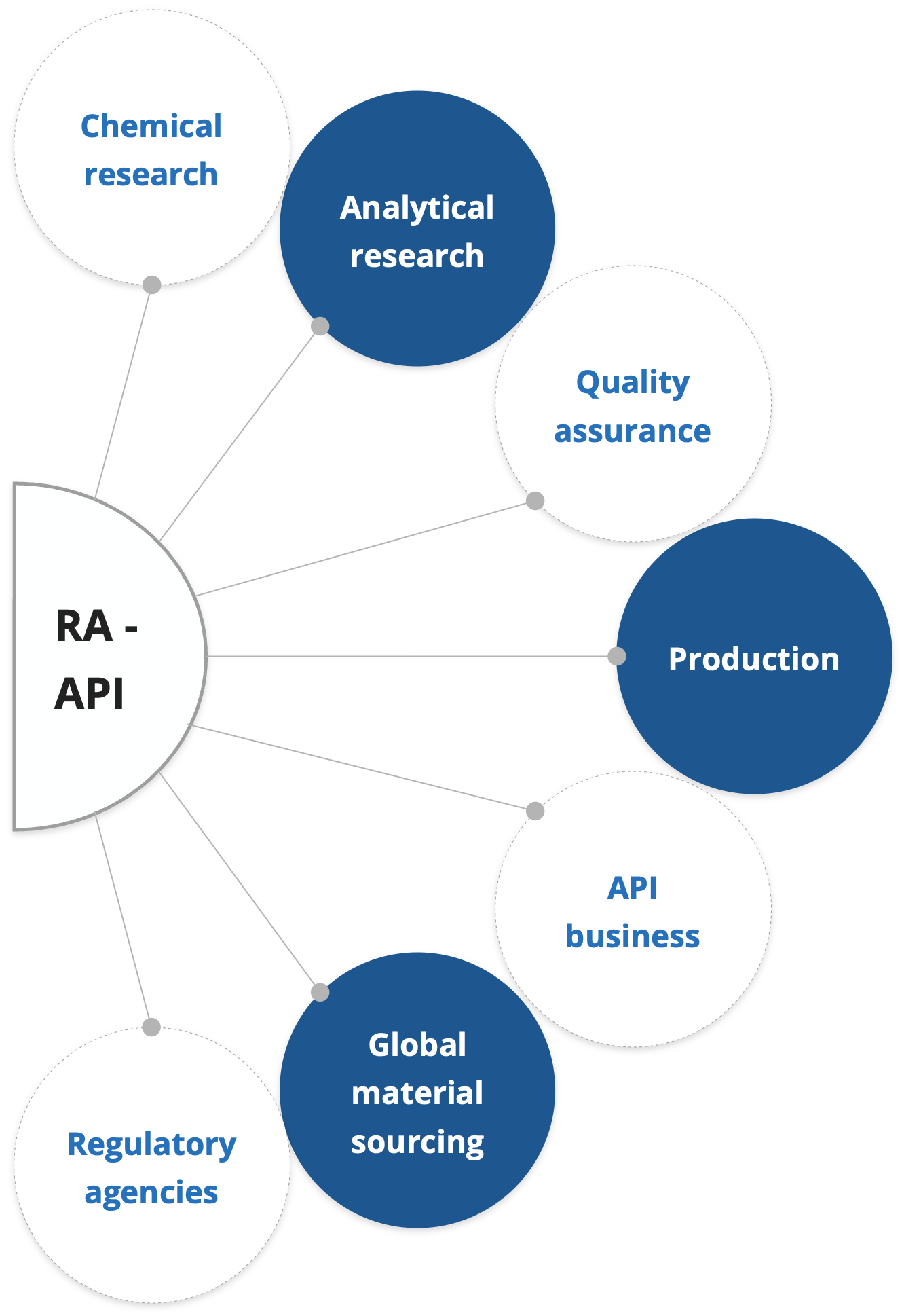
Regulatory compliance
Regulatory Affairs
Driving innovation to market success!
- Regulatory Affairs is a unique mixture of science and management to achieve a commercially important goal within a drug-development organization
- It serves as the interface between the regulatory authority and the project team, and is the channel of communication with the regulatory authority as the project proceeds, aiming to ensure that the project plan correctly anticipates what the regulatory authority will require before approving the product
- It is the responsibility of RA to keep abreast of current legislation, guidelines and other regulatory intelligence
Mission
- To obtain approval for new pharmaceutical products and ensuring that approval is maintained for as long as the company wants to keep the product on the market
- To contribute to designing the development program
- To review all documentation from a regulatory perspective, ensuring that it is clear, consistent and complete, and that its conclusions are explicit
- To provide inputs when legislative changes are being discussed and proposed
IPR
Intellectual Property:
At KIMIA, innovation lies at the core of everything we do. Our Research & Development team continuously strives to create novel, high-quality Active Pharmaceutical Ingredients (APIs) that not only meet regulatory and therapeutic standards but also set new benchmarks in efficiency, purity, and sustainability.
India Filings
We have actively protected our intellectual property through multiple patent applications filed in India. As of today, nine patent applications have been submitted, covering a range of process innovations, synthetic methodologies, and product developments across our API portfolio. These filings reflect our strong commitment to developing differentiated, value-added technologies that enhance product quality, process yield, and environmental performance.
International (PCT) Filings
To ensure global protection and scalability of our innovations, we have initiated the Patent Cooperation Treaty (PCT) filing process. These international filings extend our intellectual property coverage across key global markets, enabling us to safeguard our technologies and strengthen our competitive advantage worldwide.
Commitment to Innovation and Compliance
Our IP strategy is designed to align with our long-term vision — to be a leader in advanced pharmaceutical research while maintaining the highest standards of regulatory compliance, transparency, and ethical practice. Every innovation is documented, protected, and commercialized in accordance with global IP frameworks to ensure value creation for our partners and stakeholders.
- Important Links

KIMIA BIOSCIENCE LIMITED
974, Aggarwal Millennium Tower II,
Netaji Subhash Place, Pitampura,
New Delhi-110 034
+91-11-4706 3600
info@kimiabiosciences.com
sales@kimiabiosciences.com
Kimia Biosciences Ltd™ is a leading manufacturer of bulk drugs focused on high-potential therapeutic segments. The company is pursuing an ambitious growth strategy driven by infrastructure expansion and Contract Development & Manufacturing (CDMO) initiatives.